Free Textile Article
All about textile & FiberFree Textile Article
All about textile & FiberINTRODUCTION polyster
INTRODUCTION
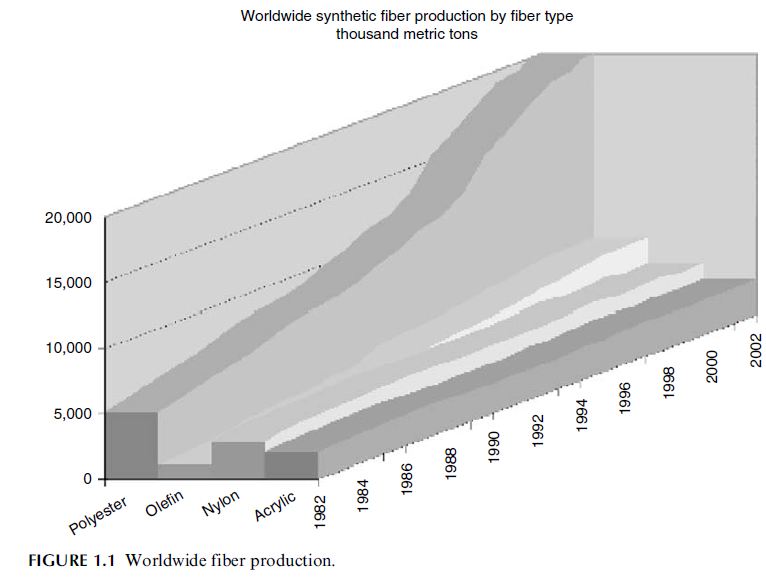
Polyester fiber, specifically poly(ethylene terephthalate) (PET), is the largest volume synthetic
fiber produced worldwide. The total volume produced in 2002 was 21 million metric tons or
58% of synthetic fiber production worldwide. The distribution of synthetic fiber production
by chemistry is shown in Figure 1.1 [1].
If one assumes the total production is a single 5 denier per filament (dpf) (~20 mm in
diameter) filament, the total length would be about 0.01 light years (~1014 m) or the
equivalent of about one million trips to the moon. While other polyesters are commercially
produced in fiber form—poly(ethylene naphthalate) (PEN); poly(butylene terephthalate)
(PBT); poly(propylene terephthalate) (PPT); and poly(lactic acid) (PLA); thermotropic polyester
(liquid crystalline polymer (LCP)—these are of insignificant volume compared to PET.
Hence this chapter focuses primarily on PET.
The reasons for the dominating success of PET fiber are:
. Low cost
. Convenient processability
. Excellent and tailorable performance
The basis of the low cost lies in the high efficiency of the conversion of mixed xylenes to
terephthalic acid, the melting temperature (2808C) being well within the range of commercial
heating fluids, and the glass transition temperature (758C), allowing the convenient stabilization
of spinline- or drawline-introduced morphology and molecular orientation. The excellent
performance results from the ability to accurately control fiber morphology (distribution and
connectivity of crystalline and noncrystalline load-bearing units), allowing the balance of
thermal and dimensional stabilities, transport, and mechanical properties to be controlled.
Over the decades, since its introduction in the 1960s, polyester technology has evolved into a
large number of products that range from cotton-blendable staple to high-performance tire
cord. It is likely that PET will continue to dominate as the synthetic fiber of choice in future,
although profitability has constantly eroded with time and production has shifted from the
United States and Europe to Asia.
Polyester fibers have been reviewed in many publications [2–4], most recently by East [5],
and the reader is directed to these publications for additional details. This work provides the
reader with an overview of polyester fiber technology, sufficient to allow the vast and detailed
open and patent literature related to polyester fibers generally, and PET fiber specifically, to
become more meaningful.
1.2 PET HISTORY
The development of PET fiber began with the pioneering work on condensation polymers led
by W.H. Carothers of DuPont in the 1930s [6].
Carothers focused on aliphatic polyesters and the resulting properties were poor compared
to the aliphatic nylons that were simultaneously explored by his group. Much improved fiber
performance was achieved in the early 1940s by the team comprising Whinfield and Dickson
[7], Calico Printers Association Laboratory in Great Britain. Their work focused on aromatic–
aliphatic polyesters from terephthalic acid (TA) and ethylene glycol. The same studies examined
other aliphatic–aromatic polyester compositions, including PBT, PPT, and PEN. Commercialization
of PET was rapid after World War II with the introduction of Terylene in Great
Britain by ICI and the introduction of Dacron in the United States. Other products soon
followed and PET successfully entered the textile market as both filament yarn and staple, and
the industrial market as a rubber reinforcement filament yarn, primarily for use in the sidewalls
of passenger car tires. Key properties were wash-and-wear characteristics in textiles and high
modulus, coupled with excellent modulus retention, in industrial applications. The detailed
review of Brown and Reinhart [8] described this history.
Polyester fiber, specifically poly(ethylene terephthalate) (PET), is the largest volume synthetic
fiber produced worldwide. The total volume produced in 2002 was 21 million metric tons or
58% of synthetic fiber production worldwide. The distribution of synthetic fiber production
by chemistry is shown in Figure 1.1 [1].
If one assumes the total production is a single 5 denier per filament (dpf) (~20 mm in
diameter) filament, the total length would be about 0.01 light years (~1014 m) or the
equivalent of about one million trips to the moon. While other polyesters are commercially
produced in fiber form—poly(ethylene naphthalate) (PEN); poly(butylene terephthalate)
(PBT); poly(propylene terephthalate) (PPT); and poly(lactic acid) (PLA); thermotropic polyester
(liquid crystalline polymer (LCP)—these are of insignificant volume compared to PET.
Hence this chapter focuses primarily on PET.
The reasons for the dominating success of PET fiber are:
. Low cost
. Convenient processability
. Excellent and tailorable performance

The basis of the low cost lies in the high efficiency of the conversion of mixed xylenes to
terephthalic acid, the melting temperature (2808C) being well within the range of commercial
heating fluids, and the glass transition temperature (758C), allowing the convenient stabilization
of spinline- or drawline-introduced morphology and molecular orientation. The excellent
performance results from the ability to accurately control fiber morphology (distribution and
connectivity of crystalline and noncrystalline load-bearing units), allowing the balance of
thermal and dimensional stabilities, transport, and mechanical properties to be controlled.
Over the decades, since its introduction in the 1960s, polyester technology has evolved into a
large number of products that range from cotton-blendable staple to high-performance tire
cord. It is likely that PET will continue to dominate as the synthetic fiber of choice in future,
although profitability has constantly eroded with time and production has shifted from the
United States and Europe to Asia.
Polyester fibers have been reviewed in many publications [2–4], most recently by East [5],
and the reader is directed to these publications for additional details. This work provides the
reader with an overview of polyester fiber technology, sufficient to allow the vast and detailed
open and patent literature related to polyester fibers generally, and PET fiber specifically, to
become more meaningful.
1.2 PET HISTORY
The development of PET fiber began with the pioneering work on condensation polymers led
by W.H. Carothers of DuPont in the 1930s [6].
Carothers focused on aliphatic polyesters and the resulting properties were poor compared
to the aliphatic nylons that were simultaneously explored by his group. Much improved fiber
performance was achieved in the early 1940s by the team comprising Whinfield and Dickson
[7], Calico Printers Association Laboratory in Great Britain. Their work focused on aromatic–
aliphatic polyesters from terephthalic acid (TA) and ethylene glycol. The same studies examined
other aliphatic–aromatic polyester compositions, including PBT, PPT, and PEN. Commercialization
of PET was rapid after World War II with the introduction of Terylene in Great
Britain by ICI and the introduction of Dacron in the United States. Other products soon
followed and PET successfully entered the textile market as both filament yarn and staple, and
the industrial market as a rubber reinforcement filament yarn, primarily for use in the sidewalls
of passenger car tires. Key properties were wash-and-wear characteristics in textiles and high
modulus, coupled with excellent modulus retention, in industrial applications. The detailed
review of Brown and Reinhart [8] described this history.