Free Textile Article
All about textile & FiberFree Textile Article
All about textile & FiberProcessing of Thermotropic Polyesters
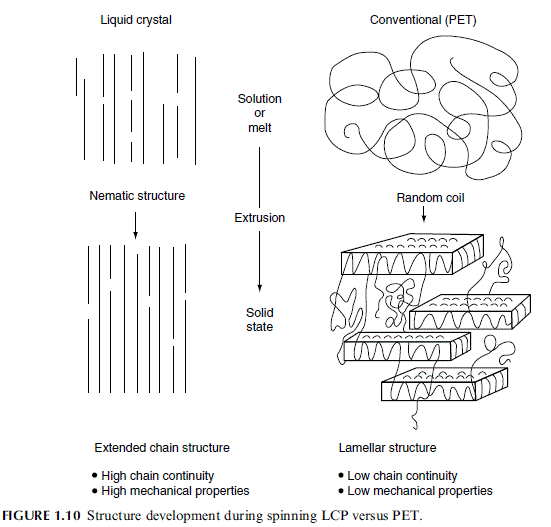
Thermotropic polyesters are melt-spun from the nematic phase and orient easily in an
elongational flow field (moderate drawdowns=forces are sufficient). In the fiber case, highly
oriented fibers form easily with an initial modulus close to theory—typical values range from
about 70 to 150 GPa. Ward [46] has shown that the tensile modulus may be described by an
‘‘aggregate model,’’ i.e., the modulus is a function of the inherent chain modulus, the
molecular chain orientation, and the shear modulus (which described the stress transfer
between chains). The tensile strength of LCP fibers follows the prediction of the ‘‘lag-shear
model’’ [47]. Both the aggregate model and the lag shear model treat the LCP as though it
elongational flow field (moderate drawdowns=forces are sufficient). In the fiber case, highly
oriented fibers form easily with an initial modulus close to theory—typical values range from
about 70 to 150 GPa. Ward [46] has shown that the tensile modulus may be described by an
‘‘aggregate model,’’ i.e., the modulus is a function of the inherent chain modulus, the
molecular chain orientation, and the shear modulus (which described the stress transfer
between chains). The tensile strength of LCP fibers follows the prediction of the ‘‘lag-shear
model’’ [47]. Both the aggregate model and the lag shear model treat the LCP as though it
ادامه مطلب ...
Chemical Structure of LCPs
Thermotropic polyester backbone chemistry is characterized by a high degree of aromaticity,
planarity, and linearity in the chain backbone. Most common moieties are p-phenylene, 1,4-
biphenyl, and 2,6-naphthalyl moieties linked by ester or amide linkages. Polymers that form
liquid crystal phases in the melt are thermotropic, whereas those that form liquid crystalline
phases in solution are lyotropic. The all-aromatic polyester homopolymers tend to be intractable,
decomposing at temperatures well below their melting points and insoluble in most
planarity, and linearity in the chain backbone. Most common moieties are p-phenylene, 1,4-
biphenyl, and 2,6-naphthalyl moieties linked by ester or amide linkages. Polymers that form
liquid crystal phases in the melt are thermotropic, whereas those that form liquid crystalline
phases in solution are lyotropic. The all-aromatic polyester homopolymers tend to be intractable,
decomposing at temperatures well below their melting points and insoluble in most

solvents. Successful melting point reduction strategies include incorporation of comonomers
to lessen crystal packing, decrease chain linearity, and increase chain-to-chain distance. All
these approaches lower the polymer melting point and, when the melting temperature is
reduced to below the polymer decomposition temperature, stable melt processing is possible.
These approaches have led to large numbers of melt processable thermotropic polyesters.
Typical LCP monomer and polymer chemistries of industrial importance are shown in
Figure 1.11. Much of the cost of LCP fibers is the result of high monomer cost and limited
monomer availability.
to lessen crystal packing, decrease chain linearity, and increase chain-to-chain distance. All
these approaches lower the polymer melting point and, when the melting temperature is
reduced to below the polymer decomposition temperature, stable melt processing is possible.
These approaches have led to large numbers of melt processable thermotropic polyesters.
Typical LCP monomer and polymer chemistries of industrial importance are shown in
Figure 1.11. Much of the cost of LCP fibers is the result of high monomer cost and limited
monomer availability.
HIGH-PERFORMANCE POLYESTER FIBERS—PEN AND LCPS
The polyester derived from ethylene glycol and naphthalene-2,6-dicarboxylic acid was first
discovered by ICI in the late 1940s [43]. It has a much higher Tg than PET and gives strong,
high-modulus fibers, but the inaccessibility of the diacid was an insurmountable problem until
recently. Now, firms like Amoco (now Solvay) are able to supply the dimethyl ester of 2,6-
NDA, and the polymer (PEN) is increasingly used in high-performance polyester films and
for high-softening-point blow-molded bottles and containers. Recently, Honeywell have
started producing a high-performance PEN fiber under the name PENTEX. This absorbs
UV light owing to the naphthalene ring. In Japan, stretch-blow-molded PEN bottles are used
to package vitamins and baby food, which would otherwise be adversely affected by UV light.
discovered by ICI in the late 1940s [43]. It has a much higher Tg than PET and gives strong,
high-modulus fibers, but the inaccessibility of the diacid was an insurmountable problem until
recently. Now, firms like Amoco (now Solvay) are able to supply the dimethyl ester of 2,6-
NDA, and the polymer (PEN) is increasingly used in high-performance polyester films and
for high-softening-point blow-molded bottles and containers. Recently, Honeywell have
started producing a high-performance PEN fiber under the name PENTEX. This absorbs
UV light owing to the naphthalene ring. In Japan, stretch-blow-molded PEN bottles are used
to package vitamins and baby food, which would otherwise be adversely affected by UV light.
ادامه مطلب ...
POLYESTER FIBERS BASED ON TEREPHTHALIC ACID
PBT was examined in detail in the early 1950s both in Europe and the United States as a
textile fiber. It had many attractive properties compared to PET; it could be melt-spun at
lower temperatures and, owing to its polymer chemistry, it was inherently whiter than PET.
As a fiber, it was much more elastic and had excellent resilience and recovery from small
deformations. It dyed easily with disperse dyes at the boil, not needing to be dyed under
pressure like PET. PBT fiber resisted the common photo-oxidative yellowing and it seemed to
have a bright commercial future. However, the reason why PBT never achieved the success of
PET in textiles was because 1,4-butanediol is significantly more expensive than ethylene
glycol. Also, PBT did not have the pleat-retaining properties of PET in blends. However, it
succeeded as a polyester carpet fiber, where its resilience and ease of dyeing were assets,
although it had to compete against nylon.
Another of the pioneer polyesters was polytrimethylene terephthalate (PTT). This was
recognized very early on as a fiber with outstanding resilience. PTT has been known in many
ways as an ideal textile fiber for over 60 years. It remained on the shelf until, in the last
decade, it became a commercial product owing to two new routes to the crucial intermediate
1,3-propanediol. One route is petrochemically derived (hydroformylation of ethylene oxide),
while the other is a fermentation route using corn sugar to make 1,3-propanediol directly
using genetically modified bacteria [40].
-
Eastman Kodak introduced a new polyester as a staple fiber called ‘‘Kodel’’ in 1958. A
new diol was introduced to derive a patent-free composition of matter; a mixture of cis- and
trans-1,4- cyclohexanedimethanol made by the exhaustive hydrogenation of dimethyl terephthalate.
This polyester had a higher Tg than PET and also a higher melting point, but it was
successful enough to find a market. In recent years, the polyester has found use in polyester
carpets [41,42].
textile fiber. It had many attractive properties compared to PET; it could be melt-spun at
lower temperatures and, owing to its polymer chemistry, it was inherently whiter than PET.
As a fiber, it was much more elastic and had excellent resilience and recovery from small
deformations. It dyed easily with disperse dyes at the boil, not needing to be dyed under
pressure like PET. PBT fiber resisted the common photo-oxidative yellowing and it seemed to
have a bright commercial future. However, the reason why PBT never achieved the success of
PET in textiles was because 1,4-butanediol is significantly more expensive than ethylene
glycol. Also, PBT did not have the pleat-retaining properties of PET in blends. However, it
succeeded as a polyester carpet fiber, where its resilience and ease of dyeing were assets,
although it had to compete against nylon.
Another of the pioneer polyesters was polytrimethylene terephthalate (PTT). This was
recognized very early on as a fiber with outstanding resilience. PTT has been known in many
ways as an ideal textile fiber for over 60 years. It remained on the shelf until, in the last
decade, it became a commercial product owing to two new routes to the crucial intermediate
1,3-propanediol. One route is petrochemically derived (hydroformylation of ethylene oxide),
while the other is a fermentation route using corn sugar to make 1,3-propanediol directly
using genetically modified bacteria [40].
-
Eastman Kodak introduced a new polyester as a staple fiber called ‘‘Kodel’’ in 1958. A
new diol was introduced to derive a patent-free composition of matter; a mixture of cis- and
trans-1,4- cyclohexanedimethanol made by the exhaustive hydrogenation of dimethyl terephthalate.
This polyester had a higher Tg than PET and also a higher melting point, but it was
successful enough to find a market. In recent years, the polyester has found use in polyester
carpets [41,42].
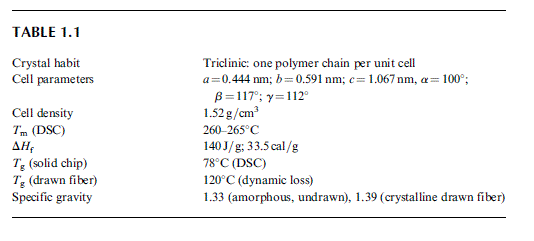
PHYSICAL PROPERTIES OF PET
PET is a semicrystalline polymer and its physical parameters have been repeatedly determined
over many years. The summary of the most recent widely accepted values [39] is shown in
Table 1.1.
over many years. The summary of the most recent widely accepted values [39] is shown in
Table 1.1.

